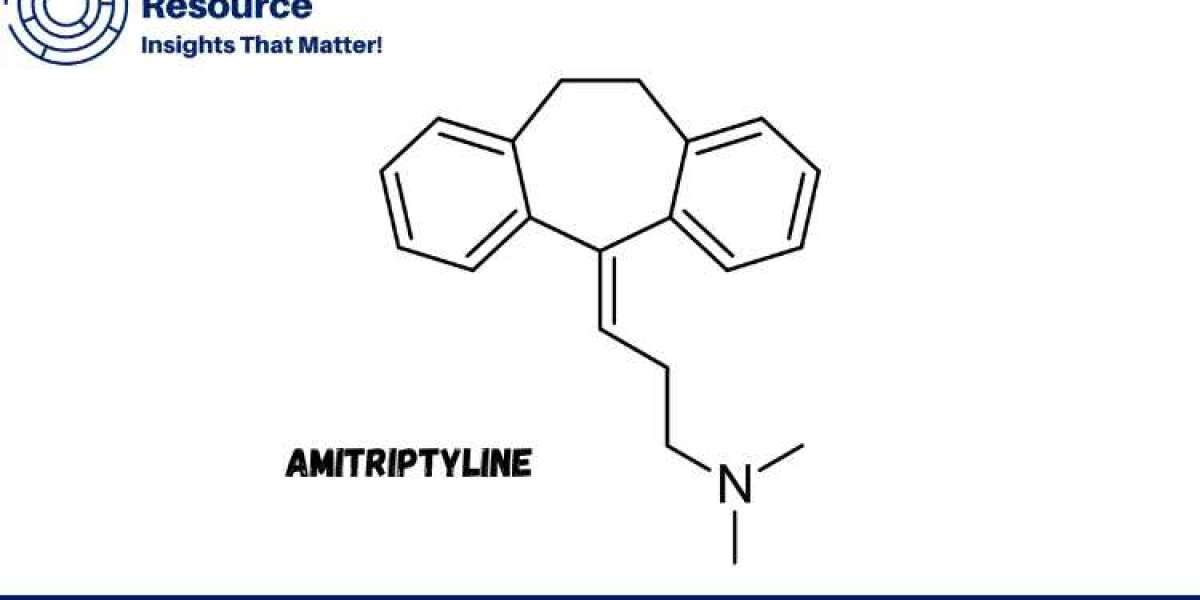
Amitriptyline is a widely used tricyclic antidepressant (TCA) prescribed for treating various mental health conditions, including depression, anxiety, and chronic pain. Understanding the Amitriptyline Production Process with Cost Analysis is crucial for pharmaceutical manufacturers and investors who seek to optimize production efficiency, ensure consistent product quality, and maintain competitive pricing in the market. This report provides a comprehensive overview of the production process, including resource procurement, market dynamics, raw material requirements, and key cost factors.
Procurement Resource Assessment Amitriptyline Production Process
The production of amitriptyline begins with a thorough procurement resource assessment to secure the necessary raw materials and intermediates for the manufacturing process. Amitriptyline is synthesized through a multi-step chemical process, which requires the procurement of high-purity starting materials and reagents to ensure the final product meets stringent pharmaceutical standards.
Request Free Sample – https://www.procurementresource.com/production-cost-report-store/amitriptyline/request-sample
The primary raw materials used in the production of amitriptyline include 10,11-dihydro-5H-dibenz[b,f]azepine (a key intermediate) and methylamine. These chemicals must be sourced from reliable suppliers who can provide consistent quality and quantity. The procurement process involves assessing supplier reliability, material availability, and pricing, as well as ensuring compliance with regulatory requirements for pharmaceutical-grade materials.
In addition to raw materials, the procurement resource assessment also includes securing the necessary equipment and technology for the production process. This may involve investing in reactors, distillation units, and purification systems, which are critical for synthesizing and refining amitriptyline. Ensuring that the equipment is up-to-date and capable of handling the specific demands of amitriptyline production is essential for maintaining efficiency and product quality.
Amitriptyline
Amitriptyline is a tricyclic antidepressant (TCA) that works by increasing the levels of certain neurotransmitters, such as serotonin and norepinephrine, in the brain. This action helps to improve mood, alleviate anxiety, and manage pain. Amitriptyline is commonly prescribed for the treatment of major depressive disorder, generalized anxiety disorder, neuropathic pain, and migraines, among other conditions.
The chemical structure of amitriptyline consists of a dibenzocycloheptene ring system with a tertiary amine side chain. This structure is responsible for its pharmacological activity, as well as its side effect profile, which can include drowsiness, dry mouth, and weight gain. Despite the availability of newer antidepressants, amitriptyline remains widely used due to its effectiveness and cost-efficiency, especially in managing chronic pain.
The production of amitriptyline involves multiple chemical reactions, starting with the synthesis of the dibenzocycloheptene core, followed by the introduction of the amine side chain. The final product undergoes purification and quality control testing to ensure it meets the required standards for pharmaceutical use.
Market Drivers
The market for amitriptyline is driven by several factors that contribute to its continued demand and production. One of the primary drivers is the prevalence of mental health conditions such as depression and anxiety, which affects millions of people worldwide. As awareness of mental health issues increases and access to healthcare improves, the demand for effective antidepressants like amitriptyline is expected to rise.
Chronic pain management is another significant driver of the amitriptyline market. Amitriptyline is often prescribed off-label for the treatment of neuropathic pain, fibromyalgia, and other chronic pain conditions. The growing number of patients seeking relief from chronic pain, coupled with the opioid crisis, has led to an increased preference for non-opioid pain management options like amitriptyline.
In addition, the cost-effectiveness of amitriptyline compared to newer antidepressants makes it an attractive option for both patients and healthcare providers. Generic versions of amitriptyline are widely available, making it an affordable treatment option in various healthcare settings, including low- and middle-income countries.
The aging population is another factor driving the demand for amitriptyline. As the global population ages, the prevalence of chronic pain, depression, and other conditions treated with amitriptyline is expected to increase, further boosting the demand for this medication.
Raw Materials Requirements
The production of amitriptyline requires several key raw materials, each of which must be carefully sourced and managed to ensure the production process runs smoothly. The primary raw materials include:
10,11-Dihydro-5H-dibenz[b,f]azepine: This intermediate is a critical building block in the synthesis of amitriptyline. It is typically synthesized in-house or purchased from specialized chemical suppliers who can provide it in the required purity and quantity.
Methylamine: This chemical is used to introduce the amine group into the dibenzocycloheptene core, forming the tertiary amine structure of amitriptyline. Methylamine must be handled with care, as it is a highly reactive and potentially hazardous substance.
Solvents and Reagents: Various solvents, such as toluene or methanol, and reagents, such as acids and bases, are required for the different steps of the synthesis process. These materials must be of high purity to ensure the quality of the final product and to minimize impurities that could affect the safety and efficacy of amitriptyline.
Catalysts: Certain steps in the synthesis of amitriptyline may require the use of catalysts to accelerate chemical reactions and improve yields. The choice of catalyst can impact the efficiency of the process and the quality of the final product.
Packaging Materials: Once the amitriptyline has been synthesized, purified, and tested, it must be packaged in a way that ensures its stability and protects it from degradation. Packaging materials may include glass vials, plastic containers, or blister packs, depending on the form in which the medication is distributed.
Costs and Key Process Information
The cost of producing amitriptyline is influenced by several factors, including raw material costs, labor, energy, equipment, and regulatory compliance. The price of raw materials, particularly key intermediates like 10,11-dihydro-5H-dibenz[b,f]azepine, can fluctuate based on supply and demand dynamics, impacting the overall production cost.
Labor costs are another significant factor, especially in regions where skilled chemists and technicians are required to manage the complex synthesis and purification processes. Automation can help reduce labor costs, but it requires a substantial initial investment in technology and equipment.
Energy costs are also a consideration, particularly for the heating, cooling, and purification steps involved in the synthesis of amitriptyline. The choice of solvents and reagents, as well as the efficiency of the equipment used, can impact energy consumption and costs.
Regulatory compliance costs must also be factored in, as pharmaceutical production is subject to stringent regulations regarding safety, quality, and environmental impact. Compliance with Good Manufacturing Practices (GMP) and other regulatory standards requires regular inspections, documentation, and adherence to strict protocols, all of which add to the overall cost of production.
Key process information includes the reaction conditions, such as temperature, pressure, and reaction time, which must be carefully controlled to ensure the desired chemical transformations occur efficiently and with minimal by-products. The purification process, typically involving recrystallization or chromatography, is also critical for removing impurities and ensuring the final product meets the required purity standards.
Looking for an Exhaustive and Personalized Report?
If you are looking for an exhaustive and personalized report that could significantly substantiate your business, our team of experts is here to provide detailed insights tailored to your specific needs. Whether you need a deeper understanding of the cost components, market dynamics, or technical specifications of the amitriptyline production process, we offer comprehensive reports that can help you make informed decisions and optimize your production process.
Our reports are designed to provide actionable intelligence that can enhance your competitive edge in the pharmaceutical market. Contact us today to learn more about how we can support your business with our in-depth industry analysis and customized reporting services.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: sales@procurementresource.com
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA